The US Court of Appeals for the Federal Circuit affirmed the Patent Trial & Appeal Board, holding that the Board did not err in finding certain challenged claims nonobvious and not unpatentable based on a showing of several objective criteria of nonobviousness and a nexus of the evidence to a commercial product embodying the claimed invention. Medtronic, Inc. v. Teleflex Innovations S.A.R.L., Case No. 21-2357 (Fed. Cir. June 05, 2023) (Moore, C.J.; Lourie, Dyk, JJ.) and Medtronic, Inc. v. Teleflex Innovations S.A.R.L., Case No. 21-2359 (Fed. Cir. June 05, 2023) (Moore, C.J.; Lourie, Dyk, JJ.)
Teleflex developed and patented a novel catheter-based stenosis intervention system that successfully mitigated long-standing risks intrinsic to existing catheter-based intervention systems, in particular damage to the coronary artery from guide catheter dislodgement or a catheter’s distal tip (i.e., the end of the catheter farthest from the insertion site). The preferred embodiments incorporated into Teleflex’s extremely successful GuideLiner products comprised a proximal substantially rigid portion (yellow), a reinforced portion (blue) and a distal flexible tip (pink), as illustrated below.
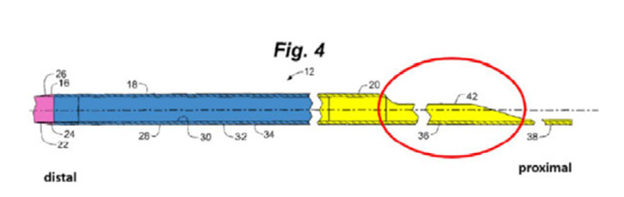
The catheters were sized so they could be inserted through standard guide catheters and thus were coined guide extension catheters. This innovative nesting feature increased guide catheter backup support while the guide extension catheter’s soft distal end was less likely to cause tissue damage once deeply inserted into patients. Teleflex’s guide extension catheters also were optimized for receiving interventional cardiological devices. This optimized function was a combination of the catheter’s coaxial lumen, that lumen’s diameter being no more than one French (i.e., 1/3 mm) less than the diameter of the guide catheter, and a proximal side opening that featured a double incline design like that illustrated above.
Teleflex’s GuideLiner was introduced in 2009 and enjoyed “undisputed commercial success and industry praise.” In 2019, Medtronic introduced its competing guide extension catheter (Telescope) and filed six inter partes review (IPR) petitions against Teleflex’s extension guide catheter family. Three of Medtronic’s petitions asserted that the challenged claims in three of Teleflex’s patents were obvious over the evacuation sheath assembly with a distal side opening used to aspirate embolic material while occluding blood flow using sealing balloons disclosed in a prior art reference (Ressemann). The other three petitions challenged claims of the other Teleflex patents as being obvious over a support catheter for delivering angioplasty balloons disclosed in a prior art reference (Kontos).
Medtronic specifically asserted that the following three elements of Teleflex’s claimed catheters were obvious:
- A proximal side opening. Medtronic argued that it would have been obvious to replace the proximal funnel structure of Kontos’s support catheter with the distal side opening of Ressemann’s evacuation sheath assembly.
- A catheter diameter that is no more than one French less than a corresponding guide catheter. Medtronic argued that in view of prior art mother-and-child dual catheter systems in which the child catheter’s diameter is no [...]
Continue Reading
read more

 Subscribe
Subscribe


