The US Court of Appeals for the Federal Circuit found that the challenged patents were invalid as anticipated and obvious in a case involving claimed ranges and prior art that included teachings with overlapping ranges. UCB, Inc. v. Actavis Laboratories UT, Inc., Case No. 21-1924 (Fed. Cir. Apr. 12, 2023) (Moore, C.J.; Chen, Stoll, JJ.)
UCB owns two prior art patents (the Mueller patents), one directed to methods for stabilizing rotigotine that covers a drug used in UCB’s Neupro® transdermal patches to treat Parkinson’s disease, and the other directed to the stable dispersions of rotigotine used in Neupro® transdermal patches.
Soon after UCB began marketing its original Neupro® transdermal patch in 2007, it discovered that rotigotine crystallized when the patch was kept at room temperature, which lowered the amount of rotigotine available to cross the skin/blood barrier and enter the patient’s circulation and reduced the product’s effectiveness. UCB recalled Neupro® from the market in the United States. In Europe, it marketed Neupro® only under “cold chain” conditions, which reduced the rotigotine crystallization.
The challenged patent in this case solved the problem of room temperature crystallization using dispersions in which the ratio of rotigotine to the stabilizer polyvinylpyrrolidone (PVP) ranged from “about 9.4 to about 9.6.” The original Neupro® formulation had a rotigotine to PVP ratio of 9:2, and the Mueller patents disclosed a partially overlapping range of 9:1.5 to 9:5, as shown in the following graphic from the Federal Circuit’s opinion:
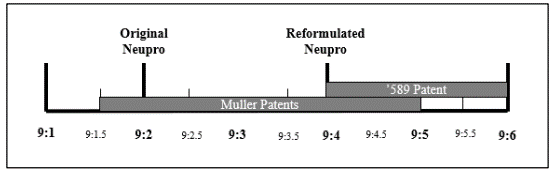
The reformulated Neupro had a ratio of 9:4 rotigotine to PVP and exhibited stability for up to two years at room temperature.
The district court held that the Mueller patents anticipated all asserted claims because a person of ordinary skill in the art (POSA) would “readily envisage” a combination of 9% rotigotine with 4% to 5% of PVP. The district court also determined that all claims were obvious in light of the Mueller patents and other prior art.
Anticipation/Overlapping Ranges
The Federal Circuit first noted that although the prior art that discloses a point within a claimed range generally anticipates that claim, such was not the case here, and the district court committed legal error treating it thus.
Instead, the Federal Circuit treated this case as one of overlapping ranges. Under that legal rubric, once a patent challenger establishes a prima facie case of anticipation by showing that the claimed range partially overlaps with the cited art, the burden shifts to the patentee to show that the “claimed range is critical to the operability of the claimed invention.” The Court stopped short of ruling that UCB had not met its burden of showing the criticality of the range because it concluded that the two patents in question were obvious in light of the overlap between the claimed ranges and those of the Mueller patents.
Teaching Away
The Federal Circuit affirmed the district court’s rejection of UCB’s arguments that Tang, another prior art reference, was the closest prior art because it did not discuss rotigotine or ratios of rotigotine to PVP, while the Mueller patents did. the Court declined to say whether the district court clearly erred when it found that the Tang reference did not teach away because it “merely expresses a preference for an optimal concentration (a 9:18 ratio)” for a formulation that did not contain rotigotine or PVP.
Unexpected Results
The Federal Circuit also affirmed the district court’s holding that UCB failed to establish unexpected results. In the Court’s view, expert testimony indicating that “small, rather than systemic, changes” to the transdermal patch formulations were needed to achieve stabilization indicated that the differences between the results obtained and those of the closest prior art were differences merely of degree, which are predictable and to be expected, versus differences in kind, in which the range would have produced “a new property dissimilar to the known property.” The evidence indicated that only minor changes in the amount of PVP were needed to address the crystallization problem, particularly in light of the success that UCB had in preventing crystallization using cold storage, which would have indicated to a POSA that fundamental changes were unnecessary.
Commercial Success
Finally, the Court affirmed the district court’s finding that there was only a weak nexus between the commercial success of the reformulated Neupro® and the claimed ratios because the Mueller patents deterred competitors from developing any competing transdermal patches.
Practice Note: This ruling clears the way for Teva and Mylan to begin marketing generic versions of Neupro®.




